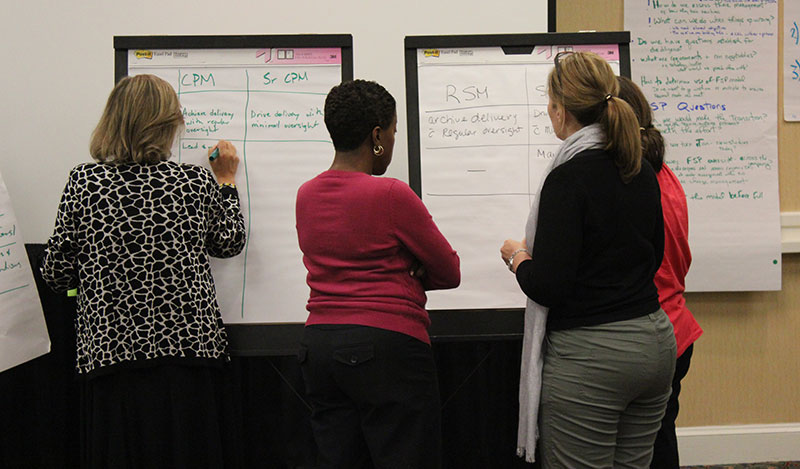The Issue:
CSL Behring is a global leader in the plasma protein biotherapeutics industry. They research, develop, manufacture and market biotherapies that are used to treat serious and rare conditions. Users of these therapies rely on them for their quality of life and, in many cases, for life itself.
The Regulatory Affairs Team spanned six sites in six countries and employed 210 people. Each RA site had its own structure and ways of working with no commonality of approach. This led to inefficiency, duplication, confusion in communicating with national health authorities, and ultimately a lack of trust in the function.
What we did:
OTM led an organizational design project which rebuilt the structure based on the team’s own definitions of what was required. Going through a comprehensive process facilitated by OTM consultants meant that no detail or consequence was left unnoticed or unresolved. Early on, the “go slow to go fast” principle was applied, resulting in a smooth implementation phase, due to complete in about a year.
The Result:
Building in the steps of Preparation, Acceptance and Commitment ensured the project delivered exactly what was needed to CSL Behring. Everything is running to schedule and efficiency and employee engagement have improved alongside a step-change in how internal and external customers view the team.
Fit for purpose, and future-proof.
Request Full Case Study
Terms: OTM values its customer relationships above all else. We distribute abridged copies of our case studies for academic use and to decision makers investigating how OTM can make an impact in their businesses. By requesting a case study, you agree 1) not to reproduce nor distribute the case study in whole or in part or 2) use the case study in any other manner.
Organization Design
Not just organization charts and personalities, organization design is about ensuring your operating model is ‘fit for purpose’ and aligned to your strategy.